Background
Since the development of proteasome inhibitors, the combination of lenalidomide (R), bortezomib (V), and dexamethasone (d) for induction has been the standard of care when treating patients with multiple myeloma. This combination has been shown to improve the clinical outcomes and quality of life of multiple myeloma patients. More recently, new generations of proteasome inhibitors, such as carfilzomib (K), and the development of monoclonal antibodies have created new opportunities for treatment that provide better outcomes with fewer side effects. The addition of daratumumab (Dara) to the RVd induction therapy has shown more potent results, increasing response rates and outcomes. However, there is a lack of evidence about the influence that age may have on the efficacy of these therapies.
Methodology
Using HealthTree Cure Hub for Multiple Myeloma (PMID: 35271305), we analyzed real-world data from 1100 patients with multiple myeloma. Patients were categorized into two groups based on age (<65 and ≥65). We compared ‘Time to Next Treatment’ (TTNT) among patients who received DaraRVd, KRd, or RVd as induction therapy. The analysis was conducted using Kaplan-Meier curves in R. Age groups were also subclassified by high-risk cytogenetics according to mSMART staging by fluorescence in situ hybridization (FISH), and the Revised International Staging System (R-ISS) stage. Chi-square tests were used to assess the association with R-ISS and mSMART stages.
Results
We analyzed the data of 1100 patients with MM, of which 718 patients (65.3%) were age <65 and 382 patients (34.7%) were age ≥65. The <65 group had a median age of 55±7, and the ≥65 group had a median age of 70±5. The majority of patients were female (n=586, 53.3%). The median year of diagnosis was 2018 (2009-2022).
In the <65 group, the majority of patients were White (88%), and other big groups were Blacks (5.8%), and Hispanics or Latinos (2.6%). Similarly, in the ≥65 group, the majority of patients were White (93.0%), and other big groups were Blacks (3.3%), and Hispanics or Latinos (2%).
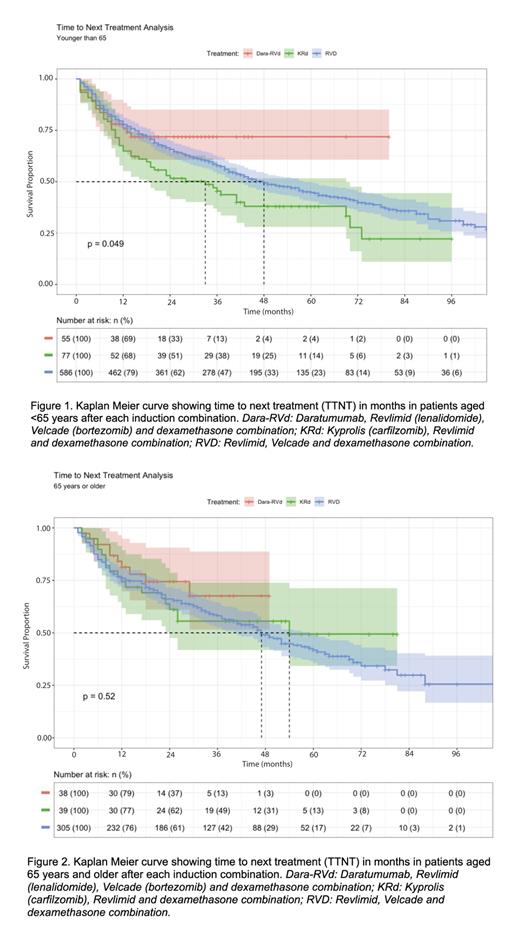
In the <65 group, 55 patients had DRVd as induction therapy, 77 KRd, and 586 RVd. In the ≥65 group, 38 patients received DRVd as induction therapy, 39 KRd, and 305 RVd.
R-ISS staging was available for 588 patients. In the <65 group, 139 patients (36.7%) were classified as R-ISS stage I, 170 patients (44.8%) stage II, and 70 patients (18.5%) stage III. In the ≥65 group, 75 patients (35.9%) were stage I, 104 patients (49.8%) were stage II, and 30 patients (14.3%) were stage III ( p=0.37). mSMART staging was available for 1035 patients, of whom patients with high-risk, double, and triple hit represented 16.2%, 5.0%, and 0.3%, respectively ( p = 0.35).
Regarding stem cell transplant (SCT), 571 patients (51.9%) underwent SCT. In the <65 group, 404 patients (56.3%) received SCT; of those, 18 (32.7%) received DRVd, 21 (53.3%) KRd, and 345 (58.9%) RVd. In the ≥65 group, 167 patients (43.7%) received SCT; of those, 12 (31.6%) received DRVd, 23 (59%) KRd, and 132 (43.3%) RVd as induction therapy.
For patients aged <65, the median TTNT was not yet reached for the DRVd group, 33 mo for the KRd group, and the RVd group was longer at 48 mo ( p=0.049). For patients aged ≥65, the median TTNT was not yet reached for the DRVd group, and 54 mo for the KRd group, which did not significantly differ from the RVd group, which had a median TTNT of 47 mo (p=0.52).
Conclusion
This study found that in the <65 age group, RVd induction therapy led to a longer TTNT compared to KRd, while in the ≥65 age group, KRd showed a longer TTNT than RVd. When considering the SCT usage rate, which increases TTNT by at least 12 months on average, KRd did not significantly improve outcomes over RVd in both age groups. However, when evaluating KRd induction, it's worth noting that KRd showed an improved outcome with a longer TTNT in the ≥65 age group compared to the <65 age group, despite toxicity concerns. These results align with the ENDURANCE trial, which also indicated that KRd did not significantly improve outcomes in standard-risk patients compared to VRd. Though the TTNT for the DRVd group is yet to be reached, the current trend suggests promising results for DRVd as a first-line therapy over KRd and RVd in both age groups. Nevertheless, further research is needed to validate and gain better insight into these observed trends.
Disclosures
Ahlstrom:Adaptive Biotechnologies: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hydren:Regeneron: Research Funding; GSK: Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Research Funding; Janssen Oncology: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal